Abstract
Background:
Use of long-term PI-based therapy can improve outcomes across treatment settings in MM. However, there are various physical, geographical, and/or socioeconomic barriers to prolonged therapy with parenteral PIs in community practice. The US MM-6 study (NCT03173092) is assessing in-class transition (iCT) from parenteral bortezomib (V)-based induction to all-oral ixazomib-based therapy with ixazomib-lenalidomide-dexamethasone (IRd) in the diverse US community population. The objective of the study is to increase the duration of PI-based treatment, while maintaining quality of life and improving outcomes. We previously reported efficacy and safety results for the first 101 US MM-6 patients (Girnius Blood 2020). Here we have analyzed updated data for this patient subset with an additional 11 months of follow-up to further evaluate efficacy and safety, and to determine reasons for premature (within 4 cycles of IRd) discontinuation.
Methods:
Transplant-ineligible/delayed-transplant (≥24 months) NDMM patients at US community sites, who had achieved stable disease or better after 3 cycles of V-based induction, received IRd (ixazomib 4 mg, days 1, 8, 15; lenalidomide 25 mg, days 1-21; dexamethasone 40 mg, days 1, 8, 15, 22) for up to 39 × 28-day cycles or until disease progression or unacceptable toxicity. The primary endpoint is 2-year progression-free survival (PFS). Rates of partial response (PR), very good PR (VGPR), and complete response (CR), and duration of therapy are key secondary endpoints. For the current analysis, sites with patients who discontinued US MM-6 were queried for more detailed information.
Results:
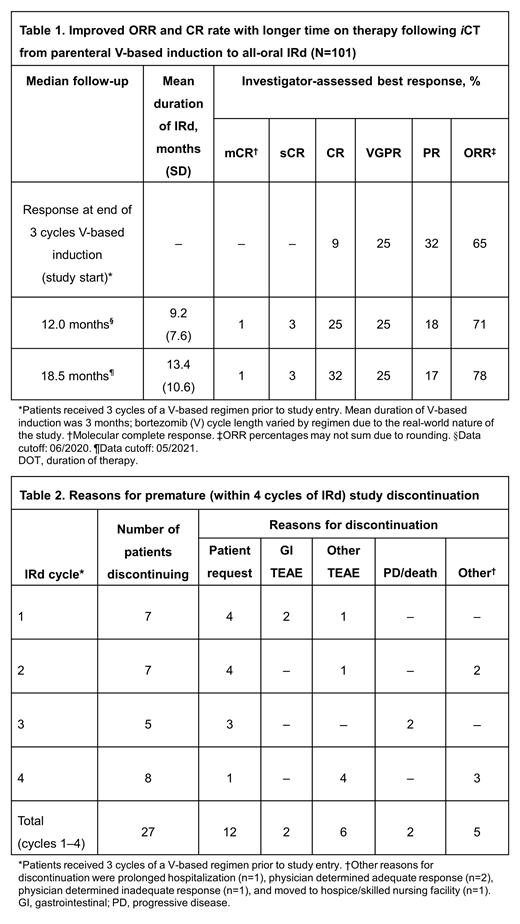
As of June 1, 2020, 101 patients had been enrolled and treated at 21 sites. Median age was 73 years (range, 48-90 years), with 81% aged >65 years; 97% had ≥1 comorbidity at the start of IRd therapy. Efficacy data after an additional 11 months of follow-up (data cut-off: May 4, 2021) showed that iCT to IRd improved responses (Table 1). Overall response rate (ORR) had improved from 65% (CR 9%, VGPR 25%, PR 32%), at the end of 3 cycles of V-based induction, to 78% (molecular CR [mCR] 1%, stringent CR [sCR] 3%, CR 32%, VGPR 25%, PR 17%) following iCT to IRd. Thirty-three patients (33%) were still ongoing on therapy at the latest data cut-off; median duration of IRd was 11.7 months and overall median duration of therapy (for all PI-based therapy, including V-based induction) was 14.6 months. At a median follow-up of 18.5 months, the 18-month PFS rate was 84%. The safety profile of IRd was consistent with previous clinical studies. Grade ≥3 treatment-emergent adverse events (TEAEs) were reported in 64% of patients and treatment-related serious TEAEs in 12% (including 4 on-study deaths), while 16% of TEAEs led to study drug discontinuation. Sixty-eight patients (67%) had completed or discontinued the study since first patient enrolment on November 15, 2017. Among these patients, 14 (21%) discontinued within 2 cycles of IRd and 27 (40%) discontinued within 4 cycles; reasons for discontinuation are presented in Table 2.
Conclusions:
With longer follow-up, use of iCT from V-based induction to IRd to achieve long-term PI-based therapy in NDMM patients demonstrates efficacy via improved response rates and acceptable PFS in this real-world setting. Over 80% of US MM-6 patients were aged >65 years and most had ≥1 comorbidity prior to study entry. The rate of patients discontinuing within 4 cycles of iCT (7 cycles of PI-based therapy in total) is concerning because these patients may not receive the full benefit of long-term PI-based treatment. To date, the majority of premature discontinuations were reported as being due to patient request (44%) followed by TEAEs (30%). Expanded site education and closer patient follow-up to reduce premature discontinuations will be addressed in the planned US MM7 iCT study in relapsed/refractory MM.
Rifkin: Coherus: Membership on an entity's Board of Directors or advisory committees; Fresenius-Kabi: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Karyopharm: Membership on an entity's Board of Directors or advisory committees; McKesson: Current Employment, Current equity holder in publicly-traded company; Amgen: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb (Celgene): Membership on an entity's Board of Directors or advisory committees. Noga: Takeda Oncology: Current Employment. Girnius: Beigene: Speakers Bureau; Takeda: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; GSK: Honoraria, Speakers Bureau; Genentech: Honoraria; Celgene: Speakers Bureau; BMS: Honoraria, Speakers Bureau. Bogard: Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited: Current Employment. Manda: Morphosys: Honoraria; Genmab: Current equity holder in publicly-traded company. Yimer: Texas Oncology: Current Employment; Pharmacyclics: Speakers Bureau; Amgen: Speakers Bureau; Sanofi: Speakers Bureau; GSK: Speakers Bureau; Beigene: Speakers Bureau; Janssen: Speakers Bureau; Astrazeneca: Speakers Bureau; Karyopharm: Current equity holder in publicly-traded company, Speakers Bureau. Whidden: Takeda Oncology: Current Employment. Birhiray: Bayer: Honoraria; Incyte: Research Funding, Speakers Bureau; Takeda: Research Funding, Speakers Bureau; Seattle Genetics: Honoraria; Coheris: Honoraria; Kyte Pharma: Honoraria; Celgene: Honoraria; Helsin: Honoraria; Amgen: Honoraria, Speakers Bureau; Pfizer: Speakers Bureau; BMS: Speakers Bureau; Tessaro: Speakers Bureau; AstraZeneca: Speakers Bureau; Alexion: Consultancy; Abbvie: Consultancy, Honoraria; Lilly: Speakers Bureau; Exelexis: Speakers Bureau; Clovis Oncology: Speakers Bureau; Sanofi Oncology: Speakers Bureau; Genomic Health: Speakers Bureau; Puma: Consultancy, Research Funding, Speakers Bureau; Pharmacyclics: Consultancy, Speakers Bureau; Jansen Bioncology: Consultancy, Speakers Bureau; Novartis: Consultancy, Honoraria, Speakers Bureau. Twumasi-Ankrah: Takeda: Current Employment. Guo: Takeda Oncology: Current Employment. Bernal-Mizrachi: Kodikaz Therapeutic Solutions: Consultancy, Current holder of individual stocks in a privately-held company, Patents & Royalties; Bigene: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Winship Cancer Institute of Emory University: Current Employment.
Real-world evaluation of long-term use of the oral proteasome inhibitor ixazomib in combination with lenalidomide and dexamethasone for the treatment of newly diagnosed multiple myeloma in non-transplant patients with stable disease after 3 cycles of a bortezomib-based induction